
【Recently, Qingdao Primedicine Pharmaceutical Technology Co., Ltd. announced that it has successfully completed the preclinical research on two indications of drug addiction and cerebral stroke, and has submitted the research results for pre-investigation of the new drug. It is anticipated that the clinical approval and the subsequent clinical phase I research will be conducted in the second half of 2020.】

Market analysis of drug abstinence indication
At present, the global drug manufacturing, trafficking, and abuse problems are becoming more prominent with the continuous expansion of sources, types, and number of drug users. The drug problem in some countries and regions continues to spread at an alarming rate. The harmful effects caused by drug usage is becoming more serious and complicated. According to data from the "Report on China's Drug Situation in 2018", by the end of 2018, there were 2.404 million drug addicts nationwide (i.e. excluding the number of people that have not been found to relapse three years after withdrawal, deaths, and emigrates), accounting to 0.18% of the total population of the country. However, the actual number of drug addicts is much higher than this number. According to reports from Western countries, the reported cases only account for 1/3 of the total number of drug addicts, which amounts to about 7.5 million drug addicts in China.
The global situation of the drug problem is even grimmer. According to statistics, about 275 million people worldwide have used drugs at least once, of which nearly 31 million are drug addicts. As the global demand for precursor chemical drugs surges and the risk of losing precursor chemicals increases, lawbreakers continue to develop and purchase non-listed regulatory chemicals as alternative precursors to evade regulatory policies. This leads to a large number of non-listed regulatory chemicals flowing into the drug-production channels. Over 60% of illegal goods and services transacted on the dark web are related to drugs and the number of transactions is rapidly growing. To top it all, the legalization of marijuana in some countries has exacerbated the spread of marijuana globally, posing strong impacts on the present international drug prohibition policies and intensifying the complexity of global drug governance.
At present, abusers of opioid drugs (such as heroin, morphine, etc.) and new synthetic drugs (such as methamphetamine, ecstasy, etc.) each account for around 50% of total drug abusers in China, of which relapsed drug takers mainly abuse synthetic drugs and cross-abuse is prominent. As of 2018, there were 312,000 addicts of mixed synthetic drugs and opioids, which was 12% of the total number of existing drug users- an increase of 16.8% year-over-year (YoY). In 2018, there was a total of 504,000 relapsed drug abusers nationwide, including 289,000 abusers of synthetic drugs, which accounted to 57.3% of the total number. The number of opioid drug abuser was 212,000, which was 42.1% of the total number. In 2016, the Drug Enforcement Administration, Ministry of Public Security, released a preliminary estimation in its report; if the drug rehabilitation cost per person is 2,000 RMB annually, then the annual turnover of the country’s drug treatment market will be as high as 15 billion RMB, which is a new and potential market for pharmaceuticals.
Market analysis of cerebral stroke indications
Cerebral stroke, also known simply as stroke, is a frequent occurring disease in middle-aged and elderly people, and is one of the three most dangerous diseases in the world today. According to the monitoring data of SinoHealth CMH, the total volume of stroke drugs in China in 2014 was 117.5 billion RMB, whereas it increased to 122.9 billion RMB in 2015 - a YoY increase of 4.6%. In 2016, the market revenue reached 129.5 billion RMB, with a growth rate of 5.4%. According to the 2018 Report on the Prevention and Treatment of Stroke in China, the number of stroke patients aged 40 or above in China reached 12.42 million. According to the 2017 China Health and Family Planning Annual Report, the total per capita hospitalization cost for stroke patients in China was 17,787 RMB; while that of patients with cerebral infarction was 9,387 RMB; and the total market volume of stroke medication in 2018 was 149 billion RMB. As the aging trend rises and living costs surge, the market size of stroke has expanded. Results of the global stroke market survey published in the New England Journal reveal that between 1990 and 2016, the overall incidence of stroke in the world had escalated. The escalation is especially prominent in East Asia, where the overall incidence increased by nearly 30%, and the rate of male patients suffering from stroke risks in their lifetime increased to 40.6% (see Figure 1). Hence, it can be concluded that cerebral stroke is gradually becoming a major disease that threatens human health.
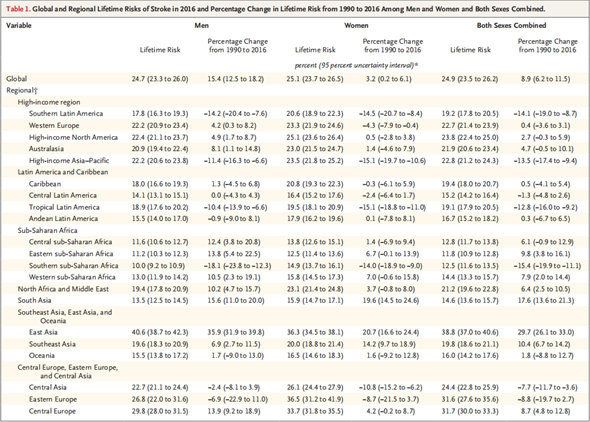
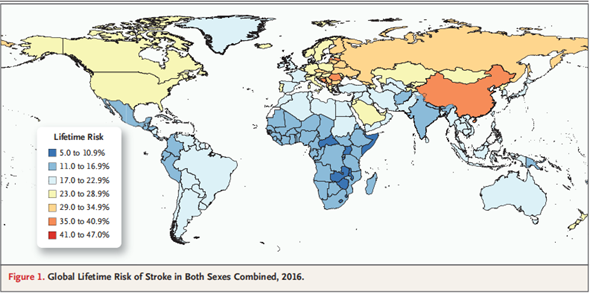
Figure 1 全球脑卒中风险评估
N Engl J Med 2018;379:2429-37. DOI: 10.1056/NEJMoa1804492
Preclinical research of PMS-001 drug abstinence indication
At present, there is no definitive medicine for drug addiction treatment in China or abroad. The main alternative therapies are opioid drugs, such as methadone, buprenorphine, and naloxone, and non-opioid drugs. However, it must be noted that these two classes of drugs pose risks of subsequent addiction and adverse reactions in patients.
The active ingredients of the drug rehabilitation medicine that has been independently developed by Primedicine Pharmaceutical are interfering peptides. This medicine can treat both opioid addiction and synthetic drug addiction. Theoretically, due to addition’s relationship with pathological learning and memory mechanism, the drug can also be applied to treat smoking, alcohol, gambling, and internet addictions without relapse. The invention is based on Long-term Potentiation (LTP) or Depression (LTD) caused by the synaptic nerve signal transmission. This treatment acts on the learning and memory mechanism by reducing the consolidation and strengthening of LTD on environmental spatial memory through blocking the hyper endocytosis of the AMPA receptor in the postsynaptic membrane. It eliminates pathological learning memory caused by drug addiction or behavioral addiction; and solves the medical and social difficulties caused by the 70% abstinence and relapse rate of opioids and new synthetic drugs.
Preclinical research of PMS-001 stroke indications
According to the characteristics of cerebral stroke, the clinical treatment strategies for ischemic stroke mainly focus on two aspects: first is treatments that improve blood supply, including thrombolysis, vasodilation, vascular remodeling, establish collateral circulation and regulate the state of blood in order to restore and promote blood supply in the cerebral ischemia area. The second is treatments that aim to protect the structure and function of nerve cells by using drugs to prevent the cascade reaction of nerve cell death, reduce the injury of nerve cells caused by ischemia, and promote the recovery of the injured nerve cells.
However, in clinical practice, the drugs available in the above two treatment strategies are extremely limited. So far, the American Food and Drug Administration (FDA) has only approved tissue type plasminogen activator (tPA) as a treatment of cerebral stroke, and no other drug for treating cerebral ischemia has been applied in clinic treatment. Japan has researched and developed Edaravone, a small molecule drug to treat cerebral ischemia. The drug uses its anti-oxidizing effect as its main mechanism. Even though the drug is being used in China, its application range is quite limited. There is an acute shortage of ischemic stroke drugs worldwide. In the actual clinical treatment process, though there is a lack of drugs for cerebral ischemic stroke, some medicines related to the pathological mechanism can still be applied according to the state of the disease, such as thrombolysis, cerebral circulation improvement, cerebral protection, anti-free radicals, antiplatelet, and blood lipid regulation, etc. When necessary, anticoagulant therapy, blood pressure regulation, intracranial pressure regulation, improvement of blood supply to the brain, cranial nerve nutrition, and symptomatic treatment can be added as well. Although the clinical application of thrombolytic drugs currently has a certain curative effect, its treatmenthas yet to achieve the desired effect due to the influence and restriction of several factors. There are three main impediments in the use of thrombolytic drugs. Firstly, the time window is narrow, which limits the number of patients being treated. Although the treatment window of tPA has been extended to 4.5 hours, the number of patients meeting the treatment conditions is still limited. Secondly, the sole effect of thrombolytic drugs cannot resolve the pathological changes after a cerebral stroke. Since tPA only dissolves thrombus and improves blood supply, it has no therapeutic effect on nerve cell injury caused by ischemia, and the disability rate of cerebral stroke remains high. Thirdly, adverse reactions such as bleeding transformation has an influence on the therapeutic effect. The most critical adverse reaction to the use thrombolytic drugs is bleeding, which can be attributed to both the direct effect of using high doses of the thrombolytic drugs, and to the pathological changes and tPA interaction in the process of cerebral ischemia. When the occurrence time of cerebral thrombosis is over 4.5 hours, the probability of hemorrhagic transformation in the ischemic area increases greatly. Upon the usage of tPA, the incidence of hemorrhagic transformation increases further, and risks the life of the patient.The above-mentioned factors directly restrict the application of tPA. Though anticoagulants are common in clinical practice, bleeding is a common adverse reaction in the treatment of acute ischemic stroke. Some anticoagulants, such as heparin, can reduce the risk of venous thrombosis, but there is an increased risk of extracranial hemorrhage, and the rate of mortality and disability of patients treated with heparin are not significantly reduced. The therapeutic effects of antithrombotic drugsand anti-platelet aggregation drugs are limited. Although antiplatelet aggregation drugs and antithrombotic drugs can improve the state of blood and reduce the incidence of thrombosis, these drugs are not competent in the treatment of acute stroke. However, they have certain significance in preventing the occurrence or recurrence of cerebral stroke. Furthermore, the clinical effects of several anti-cerebral ischemia drugs require further evaluation. Edaravone, a drug used in clinical treatment has proved to possess significant therapeutic effects and has an anti-oxidizing effect. However, large-scale experiments to verify the effects are needed, specifically to understand the duration and dosage for patients of cerebral stroke.
To summarize, there is a lack of reliable strategies for treatment and effective prevention, and drugs for treating cerebral stroke. A comprehensive study of treatment measures and methods of cerebral stroke, exploration of new prevention and treatment strategies and technologies, and development of new drugs for prevention and treatment of stroke will be a long-term research task.
A substantial number of studies have shown that the death of synaptic neuron caused by NMDA receptor overstimulation, which is induced by a cerebral stroke, is regulated by a variety of signaling pathways and related proteins, such as NMDA-NR2B/PSD95/nNOS, DAPK1, SREBP1, JNK, PTEN, Caspase-3/6, phosphorylation, ubiquitination, palmitoylation of related proteins, etc. By specifically blocking the conduction of these related pathways and protein interactions, treatment of cerebral stroke is possible.
The innovation of the compounds lies in the following aspects: (1) It has been proven that AMPA receptor hyper endocytosis is a common factor in the apoptosis of numerous pathological neurons (including Caspase-3 activation and Akt inhibition death pathway, Aβ-induced synaptic loss, and p38 MAPK/JNK death pathway mediated by the adenosine A1 receptor). Hypothetically, blocking AMPA endocytosis would have a stronger protective effect than the previous stroke drugs. (2) Based on the current clinical evaluation on the best time for stroke treatment, the most favorable treatment time is within 4.5 hours after the occurrence of a stroke. However, patients often miss the treatment time window on account of delayed diagnosis of symptoms and clinical detection. Since hyper endocytosis of AMPA receptor is the downstream link of neuronal apoptosis mediated by death signaling cascade, PMS-001, an AMPA receptor interfering peptide, can extend the treatment time window after a stroke and is beneficial in reducing the high rate of disability caused by stroke. (3) In addition to the high rate of disability, memory and cognitive impairment is also a high risk for stroke patients. With the wide application of thrombectomy, blood flow reperfusion after thrombectomy can further damage neuronal cells. At the same time, there is short-term or long-term memory loss caused by general anesthesia. However, studies have proved that synaptic plasticity of hippocampal vertebral neurons is closely related to memory and cognitive function. PMS-001, as an interfering peptide that inhibits hyper endocytosis of the AMPA receptor, can prevent synaptic Long-Time-Depression (LTD) caused by hyper endocytosis and effectively conserve memory and cognition in stroke patients.
“PMS-001 is an important project in Primedicine Pharmaceutical’s product line that produces innovative drugs that affect the nervous system. Based on previous well-researched preclinical data, the research results of the two indications, drug rehabilitation and stroke, have been submitted to the pre-Investigational New Drug (IND) program. This is only the beginning of our progress on the first-in-class research of new neurological drugs,” said Wang Yutian, founder of Primedicine Pharmaceutical and Fellow of The Royal Society of Canada (the Academy of Sciences of Canada). “Aiming at Alzheimer's disease, major depressive disorder, and myocardial infarction, the company's research and development team has achieved several international leading results in the preliminary stage of drug research and development. The abovementioned indications have also been included in the research product pipeline of the company. We look forward to the formal launch of the PMS-001 clinical trial, which signifies that the company has entered a new phase of clinical development. At the same time, preclinical studies on other projects and products in the company’s pipeline are being conducted in a systematic manner.”
Yan Yi, the CEO of Primedicine Pharmaceuticals, said, “As a pharmaceutical technology company focusing on new drug research, our goal is to build scientific and technological achievements that benefit the health of one and all. Starting with PMS-001, we hope to incessantly enrich our product assembly lines,introduce more strategic cooperation projects, promote drug listing, and provide new treatment methods for neurological disorders and more high-quality clinical practices for patients in China and the World at large by relying on our professional research and development team and leading patented technologies,”
Primedicine Pharmaceutical
Qingdao Primedicine Pharmaceutical Technology Co., Ltd. is a high-tech company emphasizing on the research and development of first-in-class new drugs, and the development and commercialization of innovative drugs related to the central nervous system in the treatment of drug addiction, Alzheimer's disease, cerebral stroke, and severe depression.
Primedicine Pharmaceutical was established in September 2016 and is situated in the beautiful coastal city of Qingdao. It is the first full-time overseas academician team in Qingdao International Academician Park. Wang Yutian, Fellow of The Royal Society of Canada, is the Chairman and the Chief Scientist of the company. The majority of the company’s team members have overseas educational background and work experience. The core members have world-class scientific research ability and extensive experience in clinical medical treatment and pharmaceutical management.
The company’s current research product pipeline includes PMS-001 (AMPA receptor modulator), PMS-002 (NMDA receptor subtype allosteric modulator), PMS-003 (SREBP-1 inhibitor), PMS-004 (JNK kinase inhibitor), etc. Most of the compounds are a result of independent research and innovation by the company’s determined scientific and technological teams, and are protected under global patents.
Website:www.primedicine.com
Copyright © 2020 Qingdao Primedicine Pharmaceutical Technology Co., Ltd. All right reserved
